Proton Therapy: A Cancer Treatment With Pinpoint Precision
Updated 19 April 2024
Proton therapy is a powerful type of cancer radiation therapy that uses high-energy proton particles to kill tumor cells and preserve critical organs. While the initial treatments were performed in physics research laboratories in the 1950s, this procedure is presently considered "new" due to substantial recent breakthroughs and its expanded scope.
Traditional radiation therapy using X-rays has conventionally and efficiently been used to treat different types of tumors. However, a large dose of the radiation is delivered to healthy tissue around the tumor which can cause symptoms such as severe malfunction of critical organs and/or mild complications (nausea, hair loss) as well as the risk of developing secondary cancers after radiation treatment. These treatments are optimized to minimize such complications, but there is always a way to improve them by evaluating new approaches, starting with selected patient cases.
Proton beam therapy uses high-energy protons that target tumors more precisely than conventional radiotherapy while avoiding healthy tissue surrounding the tumor. Subsequently, it has fewer side effects and can benefit children with cancer and patients with tumors in sensitive areas such as the brain, heart, lungs, and spine. The indications will increase as new centers and studies emerge.
What is Proton Therapy?
Proton therapy, also known as proton beam therapy, is an advanced and highly precise form of radiation therapy used to treat the tumor while reducing the dose of radiation to healthy tissue. Patients with tumors in dose-limiting organs, such as the central nervous system, benefit greatly from proton beam therapy. In pediatric cases, because everything in a child's body is sensitive, dose-limiting organs might comprise all organ systems.
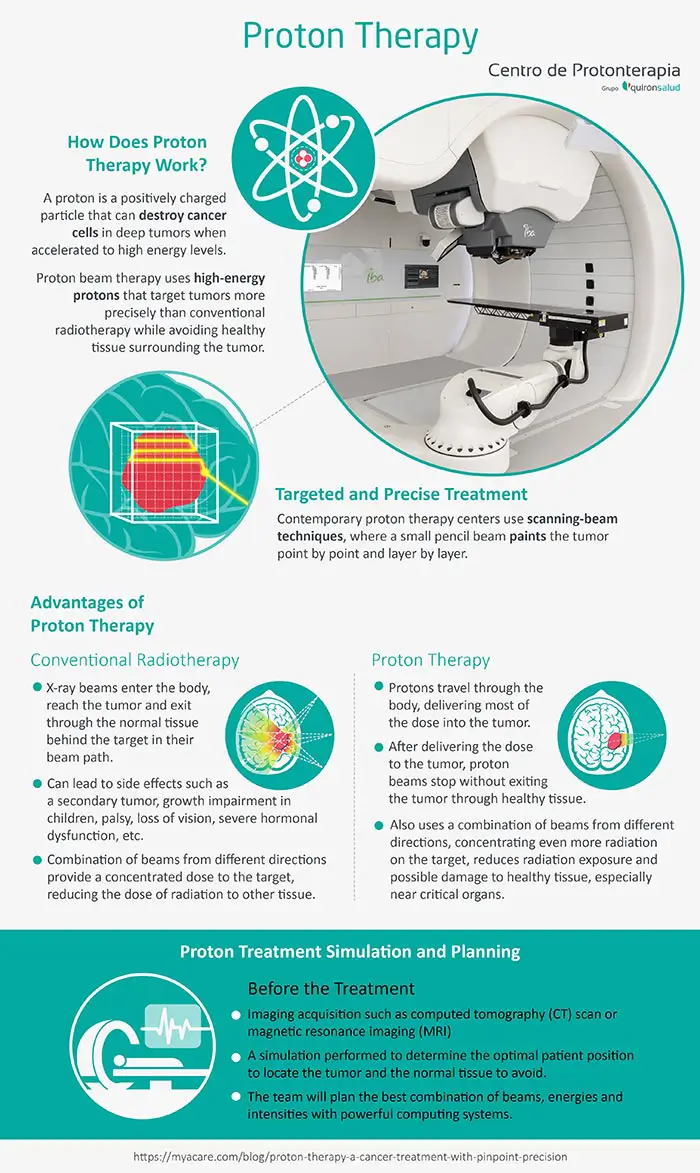
A proton is a positively charged particle that can destroy cancer cells in deep tumors when accelerated to high energy levels. A distinct aspect of protons is that they stop after the tumor due to energy loss, thus avoiding other organs. While traditional radiotherapy uses photon beams (X-rays and gamma rays), high-energy proton beams can deliver the prescribed dose with optimal precision and accuracy.
This new type of radiation treatment allows doctors to:
- Focus the proton beams on the tumor itself with less radiation exposure to the surrounding healthy tissue.
- Destroy the tumor cells layer by layer while avoiding damage to nearby non-cancerous tissue.
- Cause fewer side effects compared to conventional radiotherapy.
Proton radiotherapy can be used alone or in combination with other cancer treatments, such as conventional radiation therapy, surgery, chemotherapy, and/or immunotherapy.
It requires a multidisciplinary effort between physicists and radiation oncologists to accurately direct the beams in accordance with the size, shape, and location of the tumor.
This cutting-edge cancer treatment is not yet widely available globally (only 100 facilities worldwide). There are only two hospitals in Spain, offering Proton Therapy for Cancer Treatment.
The following infographic summarizes proton therapy, outlining how it works, the high degree of precision involved, and its advantages over conventional radiotherapy.
How Does Proton Therapy Work?
During radiotherapy, high-energy beams, like photons or protons, are delivered by a machine from outside the body to a specific volume inside the body. These beams kill cancer cells by destroying the genetic material that controls the growth and division of the cells.
In conventional radiotherapy:
- X-ray beams enter the body, reach the tumor, and exit through the normal tissues behind the target in their beam path.
- The above, can lead to side effects such as a secondary tumor, growth impairment in children, palsy, loss of vision, severe hormonal dysfunction, etc.
- A combination of beams from different directions provides a concentrated dose to the target, reducing the dose of radiation to other tissues
Unlike X-rays, protons stop at the tumor:
- Unlike photons, protons have a large mass and are charged positively, so they are slowed down as they travel through the body, delivering most of the dose into the tumor. This high release of energy can appear on a graph as a peak known as the “Bragg Peak”.
- After delivering the dose to the tumor, proton beams stop without exiting the tumor through healthy tissue.
- As with conventional radiation therapy, proton therapy also uses a combination of beams from different directions, concentrating even more radiation on the target.
- As a result, proton cancer treatment reduces radiation exposure and possible damage to healthy tissue, especially near critical organs, such as the spinal cord, brain, lungs, eyes, and heart.
Who is a Candidate for Proton Therapy?
Proton beam therapy benefits patients with tumors growing in radiation sensitive (dose-limiting) structures that are difficult to treat with conventional X-ray beam therapy. Many of these tumors require a high dose of radiation to be delivered which cannot always be fully optimized using X-ray therapy.
Among those tumors the most important ones are those growing in the:
- Brain
- Eyes (ocular orbital or para-orbital tumors)
- Base of skull and meninges
- Spine and/or paraspinal region
- Head and neck
- Lung
- Liver
- Pancreas
- Prostate
Furthermore, conventional radiotherapy in children has been associated with severe side effects, including:
- Growth and musculoskeletal complications
- Complications with endocrine functions and fertility
- Neuropsychological complications
- Secondary cancers
For these reasons, children with pediatric cancer are considered candidates for proton radiotherapy.
In addition, patients with genetic syndromes who have a high risk of developing DNA damage and secondary cancers from conventional radiotherapy are also good candidates to be considered for Proton therapy.
How is Proton Therapy Performed?
A highly specialized healthcare team carefully plans the proton radiation treatment to precisely target the tumor.
Proton treatment simulation and planning
If you are a patient prescribed a proton therapy treatment, you must know that before the treatment starts, there are usually several steps of simulation and planning involved:
- Imaging acquisition such as computed tomography (CT) scan or magnetic resonance imaging (MRI): these images and scans will help pinpoint the areas of your body that need treatment and determine the best path for the proton to reach them.
- A simulation is performed a few days before treatment, aiming to help the technical team determine the optimal patient position and immobilization to get the best ballistics to treat the tumor. You will be lying on a table or a couch, and a radiation scan will identify the exact location of the tumor on your body and the normal tissue to avoid. Patient movements should be limited during the treatment, so they may be immobilized with a mould or a mask that helps them to stay still. The type of immobilization device will depend on the location of the tumor. For example, patients with a tumor in the eye, brain, or head may be fitted with a custom-made mask.
- With this information, the team will plan the best combination of beams, energies and intensities with powerful computing systems modeling the beam and the patient with all the available information.
- During your proton therapy, you will be repositioned along the same spatial coordinates as you were during the planning scan.
Contemporary proton therapy centers use “scanning-beam techniques”, where a small pencil beam “paints” the tumor point by point and layer by layer for treatment purposes. Furthermore, high-tech radiotherapy systems such as a robotic couch, allow patients to be precisely positioned during proton beam treatment.
During proton therapy
Depending on your clinical status, you probably will receive daily treatment sessions, five days a week for 2 to 7 weeks. There are some indications, however, that may only need one or a few treatment sessions.
During proton beam therapy:
- Before beam-on, you will be placed on a treatment table or robotic chair that will help to reproduce the spatial coordinates decided at the preceding simulation/planning session.
- The medical team will make sure you are in the correct position by using laser lines to center on the anatomical marks that were placed on your body during the treatment planning scan.
- Subsequently, the medical team will start by imaging with X-ray or CT scans the regions of interest to make sure you are oriented exactly in the same position as the one decided during the simulation/planning session.
- Once the correct position is achieved using images and robots, the treatment team will leave the treatment room and head to the proton delivery controls. They are perfectly able to monitor patients when the beam is on through an audio/video system placed in the treatment room.
- When the beam is on, protons are accelerated at very high energies in a cryogenic compact synchrocyclotron machine and directed at the tumor using magnets.
Patients are helped to stay calm and still during the beam-on period to avoid any target motion out of the beam focus.
In general, each session lasts between 15-30 minutes which depends on treatment complexity, number of beams, and patient repositioning quality. Only a few minutes are used for the treatment (“beam on”), the rest is to guarantee the correct positioning.
The treatment team also ensures that weekly CT scans are done to refine the quality of the dose delivery considering possible changes in patient anatomical conditions such as weight changes and/or changes in size and shape of the tumor.
Adaptive Techniques Used in Proton Therapy
A team of imaging experts, radiation oncologists, and medical physicists collaborate to deal with challenges faced during the treatment of different patients with various conditions.
The team implements advanced adaptive techniques calibrated to each patient’s case, including:
- State-of-the-art technique in dose delivery using pencil beam scanning technology. This technology allows the intensity of the radiation to be adjusted depending on each point and layer of the tumor.
- If the patient has metallic implants, very sensitive imaging techniques and calculation methods are used to determine the best path of the protons to reduce uncertainties related to those implants.
- If the patient’s weight changes, the medical team recalculates the required ballistics of the beams to ensure that the correct dose is delivered to the tumor.
- If the patient has a tumor that is mobile, like a lung tumor when breathing, our experts synchronize the fast scanning of the beam with the patient’s respiration using devices such as spyrometers and cameras reconstructing the mobile surface of the body. Moreover, they might ask and help the patient to hold their breath for a short period of time to keep the tumor still and bombard it accurately with protons.
The radiotherapy team always conducts a comprehensive comparison between proton and photon therapy to determine the best treatment option for each patient.
Advantages of Proton Therapy
According to a large series of studies conducted by proton centers across the globe over the past few decades, proton therapy showed the following potential benefits compared to conventional radiotherapy in the following tumors sites:
- Ocular, skull base, and brain tumors (higher cure rates by escalating the dose)
- Pediatrics, head and neck, lung, breast, urological, and gynecological cancers (fewer side effects by decreasing the dose to the normal tissues)
- Breast, testicular, and lymphoma (fewer secondary cancers)
Proton beam therapy has shown great potential in successfully treating a variety of cancers in dose-limiting radiosensitive areas of the body. It helps to precisely pinpoint the location of the tumor for effective cancer treatment. This accuracy offers doctors a way to protect healthy tissue surrounding malignant tumors potentially reducing radiation induced symptoms.

Dr. Daniel Alejandro Mazal is the Technical Director and Head of Medical Physics Service at Quirónsalud Proton Therapy Center in Madrid, Spain. Dr. Alejandro was an invited scientist and visiting professor at Indiana University Cyclotron Facility and at Massachusetts General Hospital, and Harvard Medical School. Previously, he has served as Head of Medical Physics at the Curie Institute in Paris, France and as Chairman of the Particle Therapy Cooperative Group (PTCOG), a worldwide organization focused on improving radiotherapy treatment of cancer. Dr. Alejandro has over 150 research publications to his credit.

Professor Dr. Raymond Miralbell is presently the Medical Director at Quirónsalud Proton Therapy Center in Madrid, Spain. Dr. Miralbell joined the proton therapy project at the Massachusetts General Hospital and the Harvard Cyclotron Laboratory in Cambridge, Massachusetts, as clinical and research fellow (1987-1989). He is a Former Chairman of the Swiss Proton Users Group in Switzerland (1999-2007) and the Radiation Oncology Department at the University Hospital of Geneva (2007-2019). He has been a Professor at the University of Geneva, Switzerland, since 2007.

The Quirónsalud Proton Therapy Center in Madrid, Spain is one of the best of its kind in Europe. It has an excellent multidisciplinary team of renowned physicists, oncologists, and imaging experts who use specialized adaptive proton treatment techniques for patients with challenging tumors.
Sources:
Featured Blogs



